electronic configuration of bromine|full electron configuration of br : Manila Learn how to write the complete electron configuration of bromine (Br) using the Bohr model and the Aufbau principle. See the electron arrangement in different orbitals and subshells with examples and diagrams. Tingnan ang higit pa Bracken's report also highlighted a $38.8 million increase in scratch ticket grand prizes claimed so far in fiscal 2024 compared to the same period of time in fiscal 2023, $216.8 million vs. $178 .Suamuva photos & videos. EroMe is the best place to share your erotic pics and porn videos. Every day, thousands of people use EroMe to enjoy free photos and videos. Come share your amateur horny.
electronic configuration of bromine,Learn how to write the complete electron configuration of bromine (Br) using the Bohr model and the Aufbau principle. See the electron arrangement in different orbitals and subshells with examples and diagrams. Tingnan ang higit pa

The total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit paelectronic configuration of bromine full electron configuration of brScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
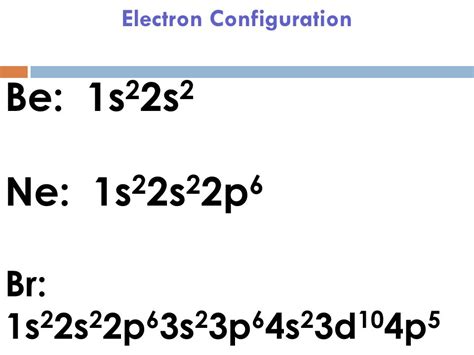
Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of bromine is 1s2 2s2 2p6 . Tingnan ang higit pa Mar 23, 2023 electronic configuration of bromine In this article today we are going to tell you about the electron configuration of Bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Please read . Learn how to write the electron configuration of bromine using the aufbau principle, periodic table, Bohr model, or orbital diagram. See examples, videos, and explanations for each method.
The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be .Electronegativities: 2.96. Atomic radius / pm: 114.5. Relative atomic mass: [79.901, 79.907] Bromine was discovered by Antoine-Jérôme Balard (FR) in 1826. The origin of .

Bromine is extracted by electrolysis from natural bromine-rich brine deposits in the USA, Israel and China. It was the first element to be extracted from seawater, but this is now .
Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. .
Learn how to write the electron configuration of bromine, a halogen with atomic number 35, using the rain method or the noble gas rule. Find out the characteristics, .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, .
The Electron configuration of bromine is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Bromine, also known as liquid fire, is defined as a chemical element that is part of the periodic table of elements. Its atomic number is 35, it is located in the group of halogens, more precisely in group VII A. Br is its atomic symbol.Bromine. Full electron configuration of bromine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. selenium ← bromine → krypton. Bromine, complete electron configuration.La configuración electrónica del bromo es: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. El bromo, también conocido como fuego líquido, se define como un elemento químico que forma parte de la tabla periódica de elementos. Su número atómico es 35, se ubica en el grupo de los halógenos, más precisamente en el grupo VII A. Br es su .To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83).The electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. How do you write the electron configuration for Bromine? The electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Signup for our weekly newsletter to get the latest news, updates and stay in the loop to find out what parents are talking .full electron configuration of brThe electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. How do you write the electron configuration for Bromine? The electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Signup for our weekly newsletter to get the latest news, updates and stay in the loop to find out what parents are talking .Bromine, element symbol Br, has an atomic number of thirty-five. One can find bromine, a halogen, in the p-block, group 17, particularly in period 4. Bromine is between chlorine and iodine, and has reactivity between the two. Bromine’s electron configuration is [Ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly .
electronic configuration of bromine|full electron configuration of br
PH0 · writing electron configurations for ions
PH1 · valence electrons of bromine
PH2 · pb 4+ electron configuration
PH3 · full electron configuration of br
PH4 · electronic structure of bromine
PH5 · electron configuration lab answers
PH6 · electron configuration chart
PH7 · br electronic configuration
PH8 · Iba pa